Septa Pharma Receives Health Canada Approval to Import Cholestyramine Amidst Canadian Shortage
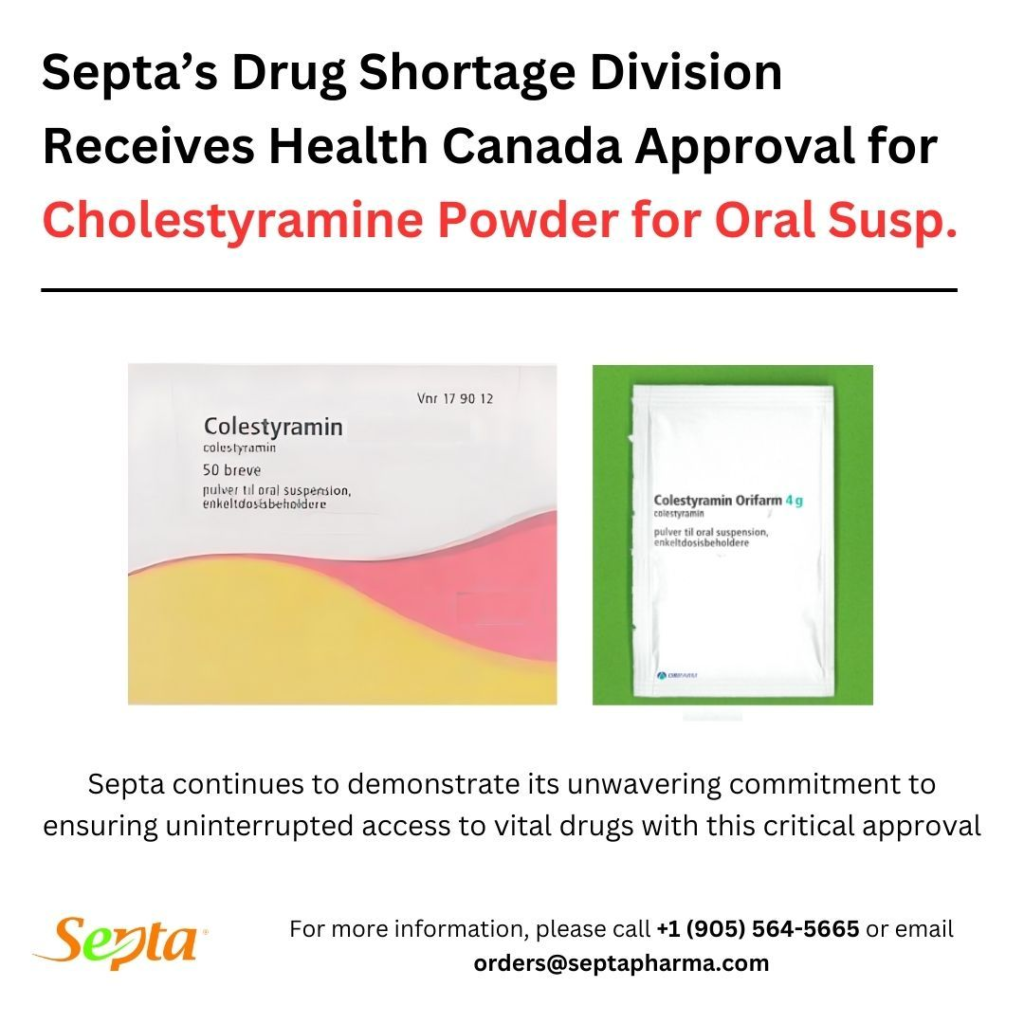
Mississauga, ON, August 2024 – Septa Pharma Inc., a leading pharmaceutical company in Canada, has announced that it has received approval from Health Canada to import Cholestyramine, a critical medication used to lower cholesterol levels and treat itching caused by a blockage in the bile ducts of the gallbladder. This approval comes at a crucial time as Canada faces a shortage of Cholestyramine, leaving many patients without access to this essential treatment.
Addressing the Shortage
The shortage of Cholestyramine in Canada has raised significant concerns among healthcare providers and patients alike. This medication is vital for managing conditions such as hypercholesterolemia (high cholesterol) and certain liver diseases. Without access to Cholestyramine, patients are at risk of serious health complications, including an increased likelihood of heart disease and a decline in quality of life due to unmanaged symptoms.
Septa Pharma’s timely intervention will alleviate the impact of this shortage by ensuring a steady supply of Cholestyramine to pharmacies and healthcare facilities across Canada. The approval from Health Canada allows Septa Pharma to import Cholestyramine from international sources, ensuring that Canadian patients receive the medications they need without further delay.
Commitment to Patient Care
Septa Pharma has a longstanding commitment to ensuring that Canadians have access to essential medications, particularly in times of shortage. The company works closely with healthcare providers, regulatory bodies, and international suppliers to bridge gaps in the supply chain, ensuring that critical medications are available to those who need them most.
“We are pleased to receive Health Canada’s approval to import Cholestyramine and address the current shortage in Canada,” said a spokesperson for Septa Pharma. “Our primary goal is to support patients and healthcare providers by ensuring that essential medications remain accessible, especially during challenging times like these.”
Looking Forward
Septa Pharma will continue to monitor the availability of Cholestyramine and other essential medications in Canada, working proactively to prevent future shortages. The company’s efforts to import Cholestyramine are part of a broader strategy to support the Canadian healthcare system by providing reliable access to critical treatments.
About Septa Pharma
Septa Pharma Inc. is a leading pharmaceutical distributor in Canada, dedicated to improving patient care by ensuring the availability of high-quality medications. With a focus on addressing shortages and meeting the needs of the healthcare community, Septa Pharma partners with global manufacturers and regulatory bodies to deliver essential drugs to Canadian patients.
For media inquiries, please contact:
Public Relations
Septa Pharma Inc.
Email: [email protected]
Phone: +1 (905) 564-5665